Allogeneic hematopoietic stem cell transplantation (alloHSCT) is an important therapeutic option, and, in some indications, the only potentially curative therapy, for the treatment of a variety of hematologic malignancies.
The success of myeloablative alloHSCT derives both from the ability to treat subjects with intensive chemo radiotherapy and from potent graft-versus-leukemia (GvL) effects mediated by donor immunity. Donor T cells in the infused stem cell graft are used to attack malignant cells, but may also strike normal host tissues and organs, resulting in graft versus host disease (GvHD). Despite the use of immunosuppressive agents, GvHD, acute or chronic, is one of the leading causes of morbidity and mortality from alloHSCT.
RGI-2001 is a liposomal formulation which contains the active pharmaceutical ingredient, KRN7000. KRN7000 is the first synthetic derivative of α-galactosylceramide (α-GalCer) compound isolated originally from the marine sponge Agelas mauritianus, which is a representative ligand for invariant natural killer (iNKT) cells. RGI-2001 activates a tolerogenic cellular pathway via iNKT cell activation, which ultimately leads to the activation of cellular cascades including regulatory dendritic cells (DCreg) and Tregs. Tregs are upregulated via an IL-4 dependent mechanism produced by iNKT cells. These antigen-specific Tregs have been shown to down regulate the immune response directed toward specific antigens without causing generalized immunosuppression while preserving graft-versus-leukemia effect (GvL).
RGI-2001 is being evaluated for the potential to reduce or prevent GvHD post allogeneic transplantation.
The aim of this analysis was to characterize the pharmacokinetic (PK) profile of RGI-7000 following weekly intravenous infusions of RGI-2001.
Methods: RGI-2001-003 is an open label, multi-center phase 2b study to evaluate the safety and efficacy of RGI-2001 for the prevention of acute GvHD in subjects following alloHSCT. Blood samples were collected for quantification of plasma concentrations of RGI-7000 predose and 0.5, 2, 4, 6, 8, 24, and 48 h after the first dose and predose, 0.5, and 4 h post dose on Day 14.
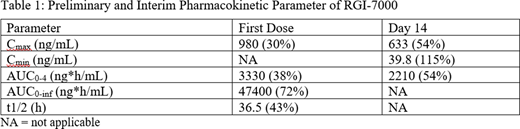
Maximum plasma concentration (Cmax), minimum plasma concentration (Cmin), area under the concentration time curve (AUC) and terminal half-life (T1/2) we calculated by non-compartmental analysis.
Results: A total of 6 subjects were evaluated in this safety phase of the study. All subjects received RGI-2001 100 µg/kg as a 30-minute IV infusion weekly for a total of 6 doses post alloHSCT. Subjects were 66.7% male, 33.3% white, 66.7% not-Hispanic or Latino. Mean age was 44 years (range 25-61 years), matched unrelated donors 83.3%. All subjects received donor PBSCT. Dose limiting toxicities (DLT) was evaluated for each subject on day 30. Mean preliminary and interim pharmacokinetic parameters (%CV) are shown in Table 1. Repeat weekly infusion of RGI-2001 lead to generally similar exposure on the first dose and Day 14. There were no deaths, no serious adverse events and no discontinuations related to study drug. There were no DLT reported. The most common treatment emergent adverse events were mucositis, pruritis/rash or erythema, decreased appetite. One infusion-related reaction (Grade 2) was reported in 1 subject and event resolved.
Conclusions: Intravenous administration of RGI-2001 resulted in quantifiable levels of RGI-7000 in all patients. Plasma levels of RGI-7000 were similar after the first dose and multiple doses. Its plasma half-life was approximately 36 hours.
Chen:AbbVie: Other: Data and Safety Monitoring Board Member; Actinium: Other: Data and Safety Monitoring Board Member; Kiadis: Consultancy; Magenta: Consultancy; Takeda: Consultancy; Incyte Corporation: Consultancy; Equillium: Other: Data and Safety Monitoring Board Member. Lane:Nanoscope Therapeutics: Consultancy; Regimmune Corporation: Consultancy; Revolution Medicines: Consultancy; Star Therapeutics: Consultancy; Valitor: Consultancy; Viewpoint Therapeutics: Ended employment in the past 24 months; Cleave Therapeutics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal